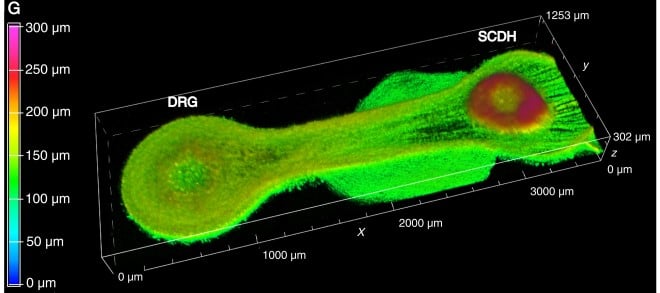
3D image of a “living circuit” in which DRG cells (left) form long extensions toward SCDH cells (right).
Identifying potential new medications is difficult and requires extensive evaluation in animal models. What’s more, usually only a small fraction of potential compounds tested are suitable for testing in humans, and researchers must screen large numbers of possible medications if they want to identify even one promising molecule.
To address this challenge, scientists funded by the Helping to End Addiction Long-term® Initiative, or NIH HEAL Initiative®, have created a tissue chip-based model system that can screen molecules in minutes.
A HEAL initiative-funded research team at Tulane University in New Orleans, together with scientists from AxoSim, Inc. in New Orleans, developed this “living pain circuit.” The team led by Michael J. Moore, Ph.D., built a 3D system of nerve cells that connect to each other just like they do in the body. This new research takes a significant step forward toward expanding medication options for the millions of Americans who need effective, non-addictive pain relief.
How Are Pain Signals Transmitted?
When we hurt ourselves, such as stepping on a sharp object without shoes, an electrical signal is triggered in specialized nerve cells near the spinal cord called dorsal root ganglion cells, or DRG cells. These cells have long extensions that receive signals from all parts of the body (such as a finger or toe) and then transmit those signals to the spinal cord. In the spinal cord, a pain signal is shuttled to other nerve cells called spinal cord dorsal horn cells, or SCDH cells, that communicate to the brain, saying “this hurts,” and then prompt a reaction – such as “lift your foot.”
Because the DRG-SCHD connection is critical for sending pain signals to the brain, it is a key target for finding new pain treatments. It is also how the body provides its own relief, through the release and action of natural opioids called endorphins.
Creating a DRG-SCDH Miniature Circuit
Moore and his team decided to increase the efficiency of identifying new pain medications by creating a model pain signaling circuit that is pared down to the absolute essentials ‒ just DRG and SCDH cells ‒ but still performs the complex signal transmission process.
To build such a living circuit, the team isolated DRG cells and SCDH cells from rat embryos and allowed each cell type to form tiny clumps in lab culture dishes. The researchers also created miniature tissue chips that had round cell chambers at the ends, connected by a narrow channel that was 2 mm long (see image). They placed a clump of DRG cells in one of the chambers, a clump of SCDH cells in the other chamber, filled the chip with a gelatin-like growth medium, and allowed the cells to grow for several weeks.
Due to the chip’s shape, the two cell types were kept apart in their respective chambers at each end, and they could only send out extensions along the connecting channel. Under these conditions, the cells created a miniature circuit just like they do in the body: each DRG cell grew one long extension that connected with SCDH cells, whereas SCDH cells only formed short, mesh-like extensions that didn’t grow toward the DRG cells.
Moore’s team also demonstrated that DRG and SCDH cells in the chips not only grew correctly but also worked as expected. They formed properly functioning nerve cell connections and, similar to what occurs naturally in the body, signals only flowed from the DRG cells to the SCDH cells, but not in the other direction.
The real test of this system, however, is whether common pain medications can inhibit signal transmission in the tissue chip model. The researchers exposed DRG and SCDH cells in the chips to the opioid morphine as well as to the non-opioid pain medications lidocaine and clonidine. All of these drugs are known to block DRG-SCDH signal transmission. Lidocaine acts mainly on DRG cells, whereas clonidine and morphine act on SCDH cells.
Each of the pain medications had similar effects on the cells in the living circuit model. Lidocaine primarily reduced release of signals from DRG cells, whereas clonidine and morphine dampened the response of the SCDH cells to DRG signals. Either way, these tests showed that the lab-grown circuit is an effective model for testing pain treatments.
Looking Ahead
These NIH HEAL Initiative-funded researchers are hoping their model system will in the future replace at least some arduous behavioral tests performed in animals that are currently in use to determine whether a potential pain medication is effective. With a living circuit, researchers can screen medication candidates in a matter of minutes and weed out those compounds that do not show desired effects. Altogether, use of this tool promises to streamline research of effective pain relief.
Another advantage of using a tissue chip system for screening pain treatments is that these lab-grown circuits can easily be produced in large numbers; as many as 50 identical circuits can be generated from a single litter of rat embryos. The team is now planning to further scale up their nerve circuit model so that researchers can simultaneously test large numbers of potential treatments in a short amount of time.
Another goal is to create a similar model with DRG and SCDH cells derived not from animals but from human stem cells. To date, most of what we know about pain signaling comes from research in animals, but differences between species exist. Comparing living circuits between animal and human cells might help researchers identify novel and effective pain treatments in humans who experience pain.
The tool would help researchers avoid wasting time and money on compounds that don’t work in humans, as well as give a second life to drugs that would normally fall by the wayside.
“The HEAL initiative’s emphasis on connecting researchers through online data repositories will be especially helpful for systems like ours to evaluate our data within the larger body of more traditional pain research,” says Moore.
He is eager for the technology to help speed the discovery of better pain treatments for the millions of patients who need them.
Read More About This Project
Read more about this project on NIH RePORTER: Human Microphysiological Model of Afferent Nociceptive Signaling and Peripheral Nerve-on-a-chip for Predictive Preclinical Pharmaceutical Testing.
National Center for Advancing Translational Sciences (NCATS)
Learn more about NCATS’ role in the NIH HEAL Initiative.
Stay Connected
Stay up to date on the latest HEAL Initiative research advances by subscribing to receive HEAL content directly to your inbox.
 U.S. Department of Health & Human Services
U.S. Department of Health & Human Services
